Merck, a science and technology company, has announced a cooperation agreement with AMCM, a German additive manufacturing company and sister company to EOS. The partnership focuses on developing 3D printing technology for tablet production that meets Good Manufacturing Practice (GMP) standards for clinical trials, with plans to expand to commercial manufacturing later.
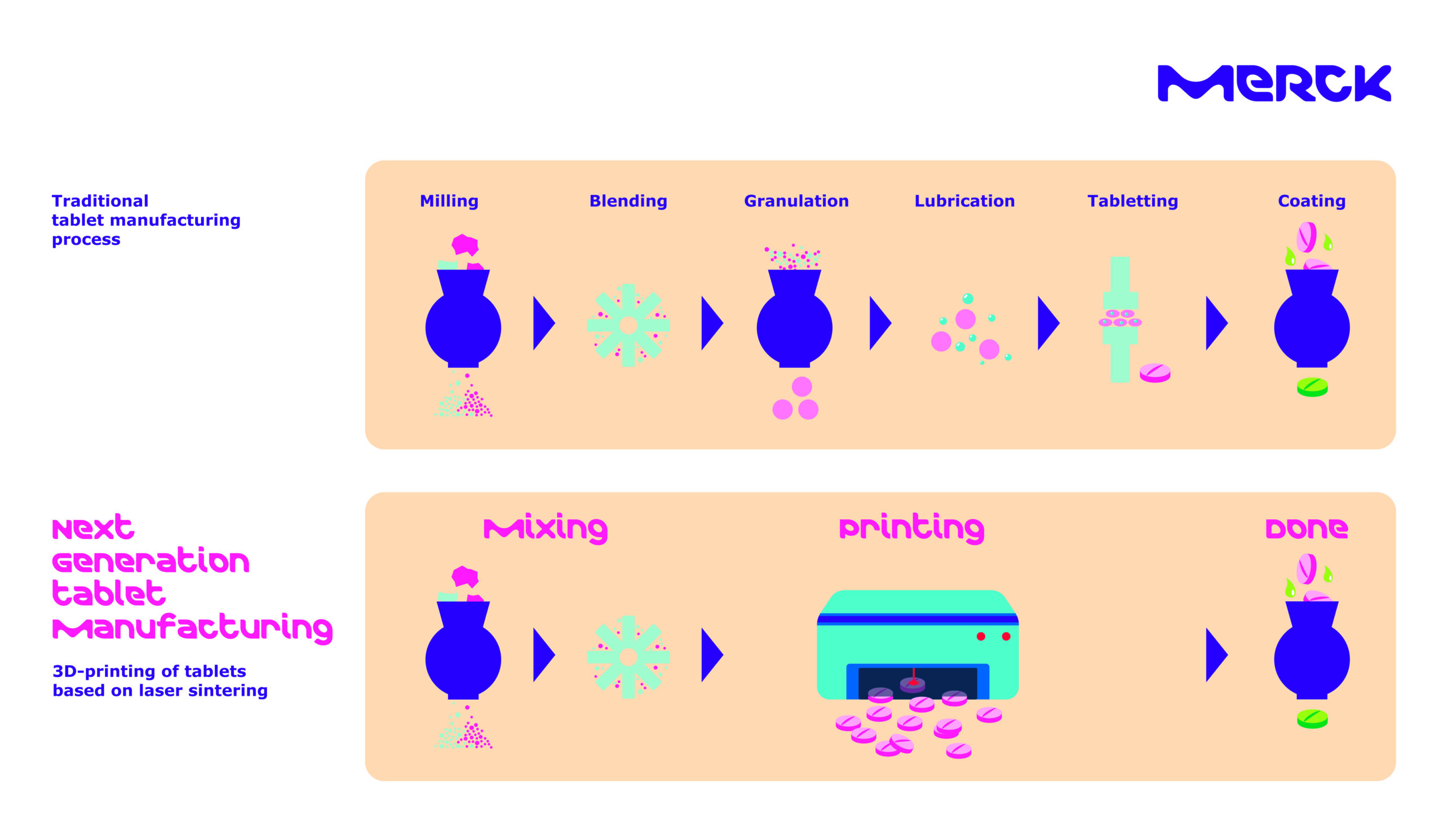
The collaboration will use powder bed fusion methods, where a laser melts and fuses powder materials layer by layer to create tablets. This approach aims to make active pharmaceutical ingredient (API) formulation more scalable while reducing the need for costly reformulations during drug development and production processes.
“Our partnership with AMCM / EOS has the potential to revolutionize the way tablets are produced. It will be a massive move towards digitalization of the industry,” said Isabel de Paoli, Chief Strategy Officer at Merck. “Our goal is to develop the industrial application of this technology, which we will make available for clinical trials first, and then move to full digital solutions at commercial scale.”
Marie Langer, CEO of EOS, added, “We are excited to support Merck on its innovation journey. This cooperation combines Merck’s formulation competences in Healthcare as well as its excipient expertise in Life Science with our long-standing additive manufacturing know-how. Together, we will help make drug development more flexible and faster.”
The companies say the technology could enable faster and less expensive tablet manufacturing. The long-term vision includes flexible local tablet production that can be adapted to specific market requirements and individual patient needs.
The project is being developed at Merck’s Innovation Center in Darmstadt, Germany, where teams work on scaling ideas that create new business opportunities across Merck’s Healthcare, Life Science, and Performance Materials divisions.
Source: merckgroup.com

