Tokyo-headquartered digital technology company Ricoh has partnered with Baltimore-based stem cell research firm Elixirgen Scientific to develop new biomedical products and services that leverage 3D bioprinting and Quick-Tissue Technology. They plan to grow the business to $1.8 billion by 2025.
According to the press release, the two companies “will support drug discovery through the manufacture and delivery of cells differentiated from induced pluripotent stem (iPS) cells, cell chips seeded with precisely differentiated cells, and evaluation services for drug responses.”
Differentiation is when an embryonic stem (ES) cell becomes specialized in order to perform a specific function, like a neuron or a heart cell. Induced pluripotent stem cells are somatic cells that have been reprogrammed to behave like ES cells by artificially “turning on” expression of specific pluripotency genes. Cell chips are simply living cells in a dish that can be used to test drug safety and efficacy before human trials.
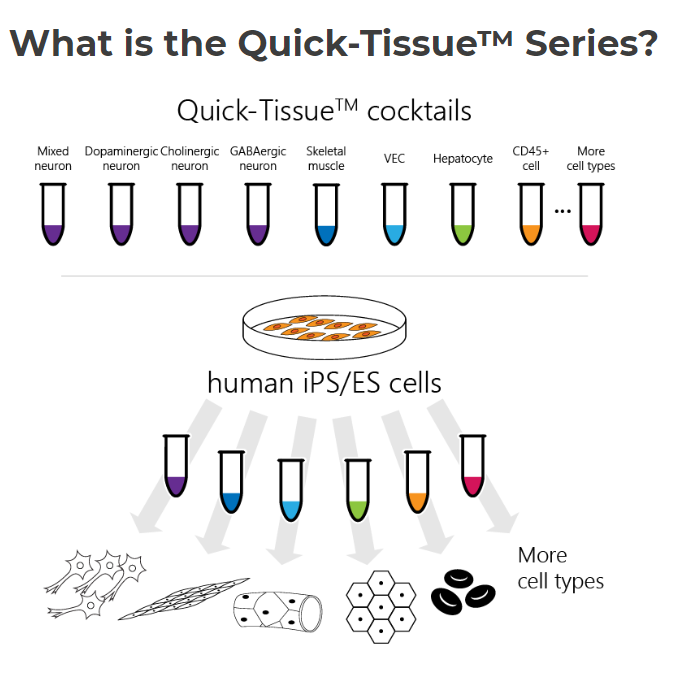
Quick-Tissue
Elixergen created the Quick-Tissue Technology that quickly differentiates iPS and ES cells into human tissues and organs, and together with Ricoh’s 3D bioprinting technology that can control the number and placement of cells, they’ll be able to rapidly produce specialized cell chips and tissues for research purposes.
stNobuhiro Gemma, Fellow and General Manager of HealthCare Business Group at Ricoh Company, stated, “We are thrilled to partner with Elixirgen Scientific on this new biomedical development initiative. By combining the technologies from our two companies, it will be possible to produce disease-specific cell chips derived from multiple iPS cell lines, for example. These cell chips can evaluate the diversity of human responses of chemicals at one time in terms of efficacy and toxicity before moving to the clinical trial stage. In the process of drug discovery, this method using the cell chips will greatly improve the entire drug development process because human diversity is considered in the earliest stage.”
In theory, disease-specific iPS cells, meaning iPS cells derived from patients with that disease, can be used as a disease model for drug screening. And differentiated cells made with their Quick-Tissue process are very similar to mature cells. As such, their future products will accurately target the phenotypes of disease cells. In other words, they should be more effective with fewer side effects.
“Ricoh has an established healthcare business with solutions such as its RICOH Regional Health Net; and in the medical imaging area, with its magnetoencephalography solution. We have also been developing technologies such as 3D Bioprinter and reference DNA plates, and with today’s announcement, this agreement establishes Ricoh firmly as a player in the biomedical field,” added Nobuhiro Gemma.