Stanford University researchers have created a computational platform that designs and 3D prints complex vascular networks needed for bioprinted organs. The system, published in Science on June 12, generates designs resembling human vascular structures 200 times faster than previous methods. This advancement addresses a key challenge in tissue engineering: ensuring oxygen and nutrients can reach all cells in artificially grown organs.
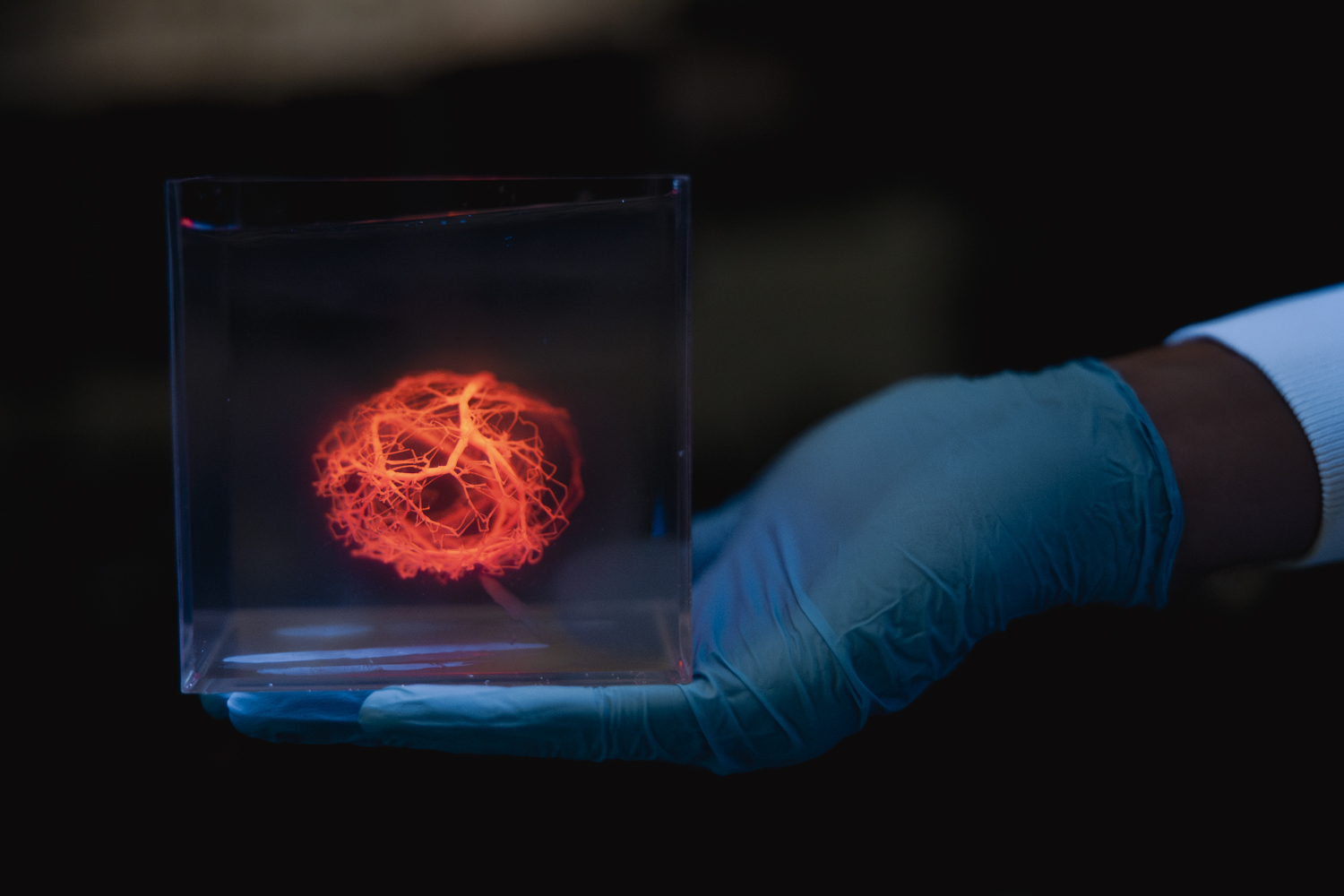
The algorithm creates vascular trees that mimic native organ blood vessel architectures while incorporating fluid dynamics simulations. “The ability to scale up bioprinted tissues is currently limited by the ability to generate vasculature for them – you can’t scale up these tissues without providing a blood supply,” said Alison Marsden, professor at Stanford Schools of Engineering and Medicine and co-senior author of the study. The design for a human heart vascular system with one million vessels took approximately five hours to generate.
Using a 3D bioprinter, the team successfully printed a network with 500 branches and tested a simpler version with human embryonic kidney cells. The researchers created a thick ring loaded with cells and built a network of 25 vessels running through it, demonstrating that the printed channels could keep cells alive when nutrients and oxygen were pumped through.
The current printed structures are channels rather than complete blood vessels with muscle and endothelial cells. “This is the first step toward generating really complex vascular networks,” said Dominic Rütsche, a postdoctoral scholar and co-first author. “We can print them at never-before-seen complexities, but they are not yet fully physiological vessels.”
The Stanford team has made their software available through the SimVascular open-source project. Researchers are now working to combine this vascular printing capability with their progress in growing heart cells from human stem cells. “We have successfully generated enough heart cells from human stem cells to print the whole human heart, and now we can design a good, complex vascular tree to keep them fed and living,” said Mark Skylar-Scott, assistant professor of bioengineering and co-senior author.
The work represents progress toward addressing the needs of more than 100,000 people on organ transplant waiting lists in the U.S. Personalized organs created using a patient’s own cells could potentially reduce both waiting times and rejection risks, though significant challenges remain before fully functional organs can be produced.
Source: news.stanford.edu

